The Science behind the
Antimicrobial Power

Through independent testing in collaboration with an award-winning biomedical laboratory, we’ve conducted a comprehensive antibacterial assessment of our Cu-Coated Polyester fabric. The results are groundbreaking: scientific research confirms that a natural and potent pathogen-fighting process occurs when copper is applied to the fabric.
The benefits of copper are well-established, and our third-party lab tests, focusing on pathogens like Staph and E. coli, have demonstrated the fabric’s proven antimicrobial properties. What’s even more remarkable is our discovery during testing—pure copper “leaches” into the polyester base, enhancing the glove’s protective power and elevating it to a whole new level of effectiveness. This unique blending and “leaching” process creates a highly effective defense mechanism against harmful pathogens, viruses, and diseases, making our gloves an extraordinary solution in the fight against dangerous microbes.
Our scientific team performed an investigational study to determine the antimicrobial properties of the copper fabric—the initial ROM based on ASTM E2180 and our copper glove material. We used a control glove material to test alongside the control material. The control and treated/test material were tested against two organisms and collected recoveries at T0 and T24. All of the samples provided were conducted in triplicate, including recovery dilution plates.
Material used in study:
Plated copper coated fabric used in conjunction wit polyester. Specification of the copper purity is Cu 99.451 / Zn 0.274 / Ni 0.073 / Cr 0.064 / Mn 0.053 / Fe 0.049 / Co 0.017 / Se 0.015. The aim was to provide screening of the material to demonstrate the qualitative antimicrobial properties of the copper-polyester glove and face-mask material.
Completion of the methods included the following work elements in two phases included:
- Start: Test ‘as is’ method in E2180 against Gram (+ ) and (-) microbe
- aureus ATCC 6538 & Pseud. aeruginosa ATCC 15442
- Require Client to Provide: test & control swatches (12 per for full
- test)
- ATCC revival/storage of microbes
- 18 hour, bacterial cultures, 3 transfers x 2 microbes
- Agar Slurry
- Sample preparation
- Bacterial Density adjustment
- Pre-wet test samples
- Inoculate slurry (final concentration ~1-5 x 10^6 cells/ml)
- Inoculate slurry on samples
- Allowed exposure time (24 hours) at incubated temp/ for microbes (RH-
- 75% or above)
- T0 dilutions/plate for count (3 ctrl + 3 treated x 2 organism x
- triplicate x 4df) = 144 plates
- Sample neutralization/recovery processing
- T24 dilutions/plate count (144 plates)
- Incubate all plates for 48 hours before final read
- Observations & Results
- Project Data Report
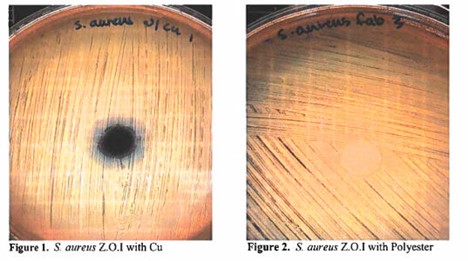
The initial phase showed that the copper fabric composition positively showed bacterial susceptibility against both a gram-positive and gram-negative organism by means of inhibition (preventing growth) and reduction of growth) reducing the numbers of organisms present within a particular environment and/or stunting growth).
Zone of Inhibition measurements including the additional “reduced density zone” observed for both sample coupons and controls.

Experimental Method
A culture of Staphylococcus aureus, ATCC # 6538 and Escherichia coli, ATCC # 25922 were initiated from a cryogenic culture that had been preserved with 10% glycerol and stored at -80 °C. These cultures went through successive passages, ending with passage number 4 (P4) that was used for testing. Tryptic Soy was used for both the liquid and solid medium. In order to generate a test inoculum of 1-5×106 cells/mL, 18-hour colonies of each organism were separately plucked from tryptic soy agar (TSA) plates and then suspended in separate conical tubes containing
2 mL of phosphate buffered saline (PBS). From these two, 2 mL inoculums, the optical density absorbances at 600nm were 0.16 for E. coli and 0.20 for S. aureus. As a guide, the 0.5 McFarland standard reads between 0.16-0.18 and corresponds to a cell density of 1-2 x 108 cells/ml, which is generally used as a starting point for the final test inoculum. ASTM E2180-18 calls for 1-5×108 cells/mL that is then diluted 1:100 in an agar slurry to yield the 1-5×106 cells/mL. Moreover, the use of an agar slurry is to provide an inoculum that does not dome which will keep the cells evenly dispersed across the entire sample surface area. Prior to testing, the optical density of the 0.5 McFarland-based culture was verified with traditional tittering via plate counting to ensure that an O.D. of 0.15-0.20 would correlate to the correct cells/mL.
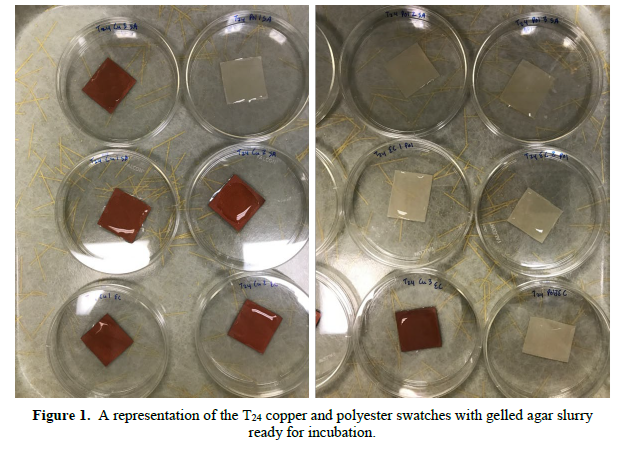
Under a BSC, both T0 and T24 designated, 3cm x 3cm swatches of the copper and polyester were aseptically inoculated with 1 mL of a 1:100 agar/bacterial slurry, one slurry for each of the different organisms. The T0 time point corresponds to the immediately recoverable harvest of the challenge inoculum. Tittering or measuring the T0 quantifies the starting inoculum concentration, which the final time point (T24) is evaluated against. The inoculated agar slurry was evenly distributed via a sterile cell scrapper and then allowed to gel on the swatches in covered petri dishes for 30 minutes.
After that time, the T24 swatches were incubated for 24 hours at 37 oC and 75% relative humidity in individually covered petri dishes (Figure 1). The T0 swatches were immediately harvested by aseptic removal with sterile forceps, placement in neutralizing/diluting PBS, followed by sonication and vortexing to dislodge any remaining viable organisms from the fabric swatch. Serial dilutions (1:10) in PBS were made and then plated (10-2-10-5) in triplicate on TSA plates. This resulted in 144 T0 plates that were incubated inverted overnight at 37 oC. Plates were assessed for colony counts 48-hours later. The T24 swatches were harvested and serial diluted in the same manner as the T0 swatches. Again, this created 144 T24 plates that were incubated and read in the same manner as the T0 swatches.
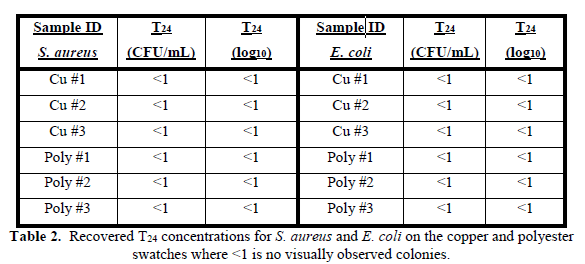
The combination of a closed dish and high level of humidity in the environmental chamber aided in preventing the agar slurry from drying out. Previous observations that contact with the copper for less than 1 hour resulted in full extirpation for both organisms provided context for predicting the results of contact for 24-hours. Similarly, to T0, we were unable to recover any colonies at the T24 for either bacteria on both materials (Table 2).
Our product becomes more powerful with every use
Polyester alone typically lacks antimicrobial properties, which led us to conduct a thorough screening of the material using advanced analytical techniques: Fourier-Transform Infrared Spectroscopy (FTIR), typically used for plastic analysis, and Energy-Dispersive X-ray Spectroscopy (EDS), used to study metals and elemental composition. FTIR analysis (Figure 3) confirmed the material was polyester, while EDS revealed that copper was present at a 0.31% weight concentration within the fabric.
It was clear that our gloves were able to eliminate microbial particles on touch and we were planning to dive deeper into the broader spectrum of pathogens and viruses however as of February 10, 2021, the EPA officially recognized that certain Copper Alloys provide long term effectiveness against viruses.
“WASHINGTON (February 10, 2021) — Today, the U.S. Environmental Protection Agency (EPA) is announcing that certain copper alloys provide long-term effectiveness against viruses, including SARS-CoV-2, the virus that causes COVID-19. As a result of EPA’s approval, products containing these copper alloys can now be sold and distributed with claims that they kill certain viruses that come into contact with them. This is the first product with residual claims against viruses to be registered for use nationwide. Testing to demonstrate this effectiveness was conducted on harder-to-kill viruses.
Read the full Press Release here: //www.epa.gov/newsreleases/epa-registers-copper-surfaces-residual-use-against-coronavirus
Please communicate for further details.
With the same research to support the basis of our product, we are proud to soon offer an entirely new patent pending PPE solution specifically designed to keep people safe, clean, healthy, and microbe-free.
If you interested in learning more about our copper gloves and facemask, please get in touch with us directly at truecopper.net or by utilizing the contact information below. We would be more than happy to connect with you to show you just what the research says regarding our copper products.
Contact information:
United States: Chris Trevino · Email: ctrevino@truecopper.net · Website: /
|